New study supports vaccination in the fight against long-COVID
Arcadia data science finds link between long-COVID prevention and vaccination.

Arcadia’s director of data science Michael Simon, pHD and chief medical officer Rich Parker, MD recently contributed important findings to the COVID-19 Patient Recovery Alliance convened by Gov. Michael Leavitt and Nancy-Ann DeParle.
Arcadia analyzed over a million deidentified patient records to uncover insights around long-COVID. What did we find?
Current vaccines reduce the risk of long-COVID even when administered up to 12 weeks after a COVID-19 diagnosis.
Here are the highlights:
- One Dose — That’s all it takes to reduce long-COVID symptoms. Patients who received at least one dose of any of the three COVID-19 vaccines (Pfizer-BioNTech, Moderna, and Johnson & Johnson) prior to a diagnosis were 7-10 times less likely to report two or more long-COVID symptoms compared to unvaccinated patients.
- 4 Weeks — The amount of time a patient has after infection to significantly reduce long-COVID symptoms via vaccination. Unvaccinated patients who received their first COVID-19 vaccination after four weeks of SARS-CoV-2 infection were 4-6 times less likely to report multiple long-COVID symptoms.
- 300% — The reduction of reported long-COVID symptoms even after receiving a vaccination up to 8 weeks after infection. Those who received their first dose 4-8 weeks after diagnosis were three times less likely to report multiple long-COVID symptoms compared to those who remained unvaccinated.
- Long-Term Protection — Vaccines work. The findings show that vaccination has a protective effect even when the first dose was administered up to 12 weeks after diagnosis.
Vaccines are safe
What we already knew
Walking into this study, Simon’s team already knew that the three COVID-19 vaccines authorized by the Food and Drug Administration are safe and effective at preventing COVID-19 infection, hospitalization, and death. Multiple clinical trials and studies have repeatedly demonstrated that fact.
What they were looking for was another benefit of vaccination – specifically, whether vaccines might reduce the likelihood of long-COVID and its associated symptoms.
Vaccines prevent long-COVID
What Arcadia’s data science team found
To put it simply: vaccines work. Arcadia’s data support the hypothesis that COVID-19 vaccination reduces the likelihood of developing long-COVID symptoms – even when the first dose is administered up to 12 weeks post-diagnosis.
The sooner patients diagnosed with COVID receive the vaccine — even a single dose — the greater chance they have to receive protection against long-COVID.
This study aids long-COVID research
What do these findings mean for public health?
The Long-COVID Study, developed in collaboration with the COVID-19 Patient Recovery Alliance, is intended to offer useful context for policy development as well as preliminary data for further exploration and hypothesis formation.
Arcadia offers these findings to the medical community in support of the fight against long-COVID, which may impact 1 in 10 patients who contract COVID-19.
A deeper dive into the data
Arcadia Research Data includes de-identified, HIPAA-compliant RWD on a growing active patient population. This data asset contains data from electronic health record (EHR) systems, practice management systems, healthcare payer claims and eligibility data, care management and clinical assessment data, and other third-party sources.
Who was included in the study?
For the purposes of this analysis, all patients needed to meet the following criteria:
- The patient must have been alive as of March 1, 2020
- The patient must have had at least one encounter with a provider documenting patient history prior to January 1, 2020
- The patient must have had at least one encounter with a practitioner who evaluated their health status after January 1, 2020
Qualifications needed to have been met by May 31, 2021, the cutoff for the data extract. This yielded a data sample covering roughly a year’s worth of COVID-19 activity in the United States, about six months of vaccination activity, and a consistent snapshot of the pandemic prior to the emergence of the delta variant (B 1.617.2) as the predominant variant in the United States at the end of June 2021.
How many patients met the criteria?
Given the initial population inclusion criteria, 25,804,278 persons in the Arcadia Data Research dataset were considered eligible for this study.
Between February 2020 and May 2021, 1,065,626 of those persons were diagnosed with COVID-19. Of those patients, 240,648 met the additional inclusion criteria of qualifying provider encounters both before January 1, 2020 and at least 20 weeks after their first COVID-19 diagnosis.
Of these patients:
- 220,460 (91.6%) had not received any vaccine against COVID-19 prior to 12 weeks after their COVID-19 diagnosis
- 8,055 (3.3%) received their first dose prior to diagnosis
- 12,133 (5.0%) were vaccinated within the first twelve weeks after COVID-10119 diagnosis
What were the specific results of the study?
Based on the results of this study, even a single dose of any of the three COVID-19 vaccines had a protective (<1.0) odds ratio relative to a patient who had no record of vaccination if vaccines were administered up to 12 weeks after diagnosis, and this protective effect was stronger the earlier the first dose occurred relative to COVID-19 infection.
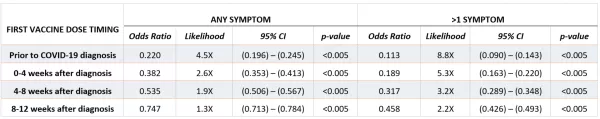
This result was supported by the general linear model, which also characterized vaccination as having a strongly negative effect on the likelihood and number of long-COVID symptoms.
Furthermore, even patients whose first vaccination occurred within 12 weeks after COVID-19 diagnosis were significantly less likely to have long-COVID symptoms than if they had remained unvaccinated. This finding would be consistent with the hypothesis that there may be residual lingering SARS-CoV-2 virus in body compartments for which a vaccine could accelerate sterilizing immunity or in some way reset the immune response.
Just The Beginning
Arcadia’s study enhances CDC’s vaccine recommendations.
Currently, CDC recommends vaccination after infection, but there is no specific recommendation of the time frame for administration, nor any mention of potential preventative effects on the development of long-COVID.
The results of Arcadia’s study suggest an added benefit to post-infection vaccination, and further indicate that the earlier vaccine is administered, the more significant the benefit.
If these results are substantiated, significant public health benefits could be realized, since up to 30% of those infected with SARS-CoV-2 developed prolonged symptoms, with many of these being debilitating and lasting for months.
Our work with the COVID-19 Patient Recovery Alliance
Support the fight against long-COVID.
The road to prevent long-COVID is just beginning, but our data indicates that vaccines help protect against adverse symptoms.
We believe this work is crucial to public health, the fight against COVID-19, and the growing concern around symptoms associated with long-COVID. We are grateful for the opportunity to contribute to the work being done by the COVID-19 Patient Recovery Alliance, and we encourage you to follow and share our group’s policy recommendations and research.
